Mansoura Veterinary Medical Journal
Editorial Policies
The Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals by the International Committee of Medical Journal Editors (ICMJE) have been adopted by MVMJ.
When a manuscript is submitted to an MVMJ, it is assumed that all authors have reviewed it and are in agreement with its contents and that it complies with all of the journal's guidelines.
Affiliations
- All authors are responsible for including their institutional affiliations, which should indicate who funded the study or supported the research.
- Your current affiliation institution is required for non-research articles.
- If you've moved since the research was done but before the article was published, be sure to include both your old and new institutional affiliations.
- Indicate that you are currently working independently if you do not have a relevant institutional affiliation.
Appeals and Complaints
All complaints, concerns, or appeals regarding authorship issues or the peer-review process, including concerns raised post-publication, should be addressed to the Editors-in-Chief, who shall investigate the claims by first, requesting information from all parties involved and second, proposing a course of action in line with academic ethical principles as outlined by the Committee on Publishing Ethics (COPE). Submissions can be halted in the review or publication process until the issues are resolved. In situations, when Editors-in-Chief are involved in the complaint, the Editorial Board members, led by the most senior member, investigate the claims and propose a course of action.
Acknowledgment
Individuals who participated in the development of a manuscript but do not qualify as an author should be acknowledged. Organizations that provide support in terms of funding and/or other resources should also be acknowledged.
Authorship
Including authors' names in an article is a crucial way to give credit to those who have contributed significantly to the work. It also ensures transparency for those accountable for the content's integrity.
Article authors must meet all of the following requirements:
- Contributed significantly to the work reported, whether in conception, study design, execution, data collection, analysis, or interpretation, or in all of these areas.
- Have drafted or written the article, substantially revised it, or reviewed it critically.
- Have decided which journal the article will be submitted to.
- Reviewed and approved all versions of the article before submission, during revision, the final version accepted for publication, and any significant changes made during proofreading.
- Agree to accept responsibility and accountability for the article's content and to participate in resolving any questions regarding the accuracy or veracity of the published work.
Before or after publication, any changes in authorship must be approved by all authors, including those being added or removed. The corresponding author is responsible for obtaining confirmation from all co-authors and providing a thorough explanation of why the change was necessary. If an authorship change is necessary after the article has been published, this will be updated via a post-publication notice. Any changes in authorship must adhere to our authorship criteria, and requests for significant authorship changes after an article has been accepted may be denied if clear reasons and evidence of author contributions cannot be provided.
Authorship Criteria
Authorship credit should be based only on substantial contributions to each of the three components mentioned below:
- Concept and design of study or acquisition of data or analysis and interpretation of data;
- Drafting the article or revising it critically for important intellectual content; and
- Final approval of the version to be published.
Participation solely in the acquisition of funding or the collection of data does not justify authorship. General supervision of the research group is not sufficient for authorship. Each contributor should have participated sufficiently in the work to take public responsibility for appropriate portions of the content of the manuscript. The order of naming the contributors should be based on the relative contribution of the contributor towards the study and writing the manuscript. Once submitted, the order cannot be changed without the written consent of all the contributors. The journal prescribes a maximum number of authors for manuscripts depending upon the type of manuscript, its scope, and the number of institutions involved (vide infra). The authors should provide a justification if the number of authors exceeds these limits.
Contribution Details
Contributors should provide a description of contributions made by each of them toward the manuscript. The description should be divided into the following categories, as applicable: concept, design, definition of intellectual content, literature search, clinical studies, experimental studies, data acquisition, data analysis, statistical analysis, manuscript preparation, manuscript editing, and manuscript review. The authors' contributions will be printed along with the article. One or more authors should take responsibility for the integrity of the work as a whole from inception to published article and should be designated as 'guarantors'.
Citations
Research and non-research articles must cite relevant, timely, and verified literature (peer-reviewed, where appropriate) to support any claims made in the article.
You must avoid excessive and inappropriate self-citation or prearrangements among author groups to inappropriately cite each other’s work, as this can be considered a form of misconduct called citation manipulation. Read the COPE guidance on citation manipulation.
If you’re the author of a non-research article (e.g. a Review or Opinion) you should ensure the references you cite are relevant and provide a fair and balanced overview of the current state of research or scholarly work on the topic. Your references should not be unfairly biased toward a particular research group, organization, or journal.
If you are unsure about whether to cite a source you should contact the journal editorial office for advice.
Conflicts of Interest/ Competing Interests
All listed authors must declare any competing interests relevant to, or which can be perceived to be relevant to the article. A competing interest can occur where the authors (or their employer, sponsor, or family/friends) have a financial, commercial, legal, or professional relationship with other organizations, or with the people working with them which could influence the research or interpretation of the results.
Competing interests can be financial or non-financial in nature. To ensure transparency, any associations that can be perceived by others as competing interests must also be declared.
Examples of financial competing interests include (but are not limited to):
- Employment or voluntary involvement
- Collaborations with advocacy groups relating to the content of the article
- Grants from an entity paid to the author or organization
- Personal fees received by the author/s as honoraria, royalties, consulting fees, lecture fees, testimonies, etc
- Patents held or pending by the authors, their institutions or funding organizations, or licensed to an entity whether earning royalties or not
- Royalties being received by the authors or their institutions
- Stock or share ownership
- Benefits related to the development of products as an outcome of the work
Examples of non-financial competing interests include (but are not limited to):
- Receipt of drugs, equipment, or access to data by an entity that might benefit or be at an advantage financially or reputationally from the published findings.
- Holding a position on the boards of industry bodies or private companies that might benefit or be at an advantage financially or reputationally from the published findings.
- Writing assistance or administrative support from a person or organization that might benefit or be at an advantage from the published findings.
- Personal, political, religious, ideological, academic, and intellectual competing interests are perceived to be relevant to the published content.
- Involvement in legal action related to the work.
All authors of a manuscript submitted to the journal will be required to complete a competing interest declaration which will be listed in the Disclosure section at the end of the article. If an author is in doubt over whether they need to disclose a competing interest, they should consult with their institution or the journal Editor, who can guide them on the right course of action.
If there are no competing interests to declare, the following statement will be added to the article “The authors declare that they have no competing interests.”
Sponsorship of clinical trials
Authors employed by pharmaceutical companies or other organizations that sponsor clinical trials must declare this as a competing interest.
Authors should adhere to the Good Publication Practice guidelines for pharmaceutical companies (GPP3), which guides to ensure responsible and ethical standards are maintained.
Corrections, expressions of concern, and retractions
Corrections may be made to a published article with the authorization of the editor of the journal. Editors will decide the magnitude of the corrections. Minor corrections are made directly to the original article. However, in cases of major corrections, the original article will remain unchanged, while the corrected version will also be published. Both the original and corrected versions will be linked to each other. A statement indicating the reason for the major change to the article will also be published. When necessary, the retraction of articles will be done according to COPE retraction guidelines. This can be in the form of a Correction notice (Corrigendum or Erratum), an Expression of Concern, a Retraction, or in rare circumstances, a Removal. The purpose of this mechanism of making changes that are permanent and transparent is to ensure the integrity of the scholarly record.
All retractions issued at the journal will ensure:
- The retraction and original article are linked in both directions
- The retracted article is clearly identified
- The original HTML version will remain, with both the HTML and PDF of the original article digitally watermarked ‘Retracted’
- A clear explanation giving the reason for the retraction is provided
- The person(s), for example, the authors and/or the Editor, who requested the retraction is clearly stated
The journal recognizes the purpose of a retraction is to correct the literature and ensure the integrity of the publication record. They are not intended as a means of punishment for authors.
Retractions will not normally be issued to resolve authorship disputes. The preferred option in this situation is to issue a corrigendum. This is provided the authors can justify the change in authorship, and this usually requires the support of their respective institutions.
To help minimize the impact of incorrect or misleading publications, all efforts will be made to issue retractions as soon as possible.
In some cases, an Expression of Concern notice may be considered where concerns of a major nature have been raised (e.g. serious research or publication misconduct), but where the outcome of the investigation is inconclusive or where due to various complexities the investigation will not be complete for a considerable time. When the investigation has been completed a Retraction or Correction notice may follow the Expression of Concern, and alongside the original article, all will remain part of the permanent published record.
A Removal notice will be issued in very rare circumstances where the problems cannot be addressed by a Retraction or Correction notice. Examples include where the content in the article is defamatory infringes on other legal rights or is subject to a court order. In the rare case of an article being removed from the journal Online, a removal notice will be issued in its place.
Consent for Publication
For all manuscripts that include details or images relating to an individual person, written informed consent for the publication of these details must be obtained from that person (or their parent or legal guardian in the case of children under 18). The consent must be for publication of their details under the Creative Commons Attribution License 4.0 (such that they will be freely available on the internet). If the person has died, consent for publication must be obtained from their next of kin. The manuscript must include a statement that written informed consent for publication was obtained.
Authors can use the consent form to obtain consent for publication, or a consent form from their own institution or region if appropriate. The consent form must state that the details/images will be freely available on the internet and may be seen by the general public. The consent form must be made available to the Editor if requested and will be treated confidentially.
Confidentiality
Manuscript submissions should be treated as private information. Manuscripts submitted to academic journals will be kept confidential and seen only by those who have a direct role in the publication process (if accepted). The editorial team consists of the editors, potential reviewers, reviewers, and the editors themselves. However, if misconduct is suspected, the manuscript may be shared with the relevant ethics committee members and institutions/organizations in order to resolve the situation. When publishing research findings, scholarly journals must use the relevant COPE flowcharts. A manuscript submission should be treated as private information. Manuscripts submitted to academic journals will be kept confidential and seen only by those who have a direct role in the publication process (if accepted). The editorial team consists of the editors, potential reviewers, reviewers, and the editors themselves. However, if misconduct is suspected, the manuscript may be shared with the relevant ethics committee members and institutions/organizations in order to resolve the situation. When publishing research findings, scholarly journals must use the relevant COPE flowcharts.
Copyright Policy
User Rights
MVMJ is an open-access journal. Users have the right to read, download, copy, distribute, print, search, or link to the full texts of articles under the following conditions: https://creativecommons.org/licenses/by/4.0/
Copyright statement stated here and embedded in each published article
Author Rights
The journal allows the author(s) to hold the copyright, and retain publishing rights without any restrictions.
Data falsification/fabrication
Where deliberate action has been taken to inappropriately manipulate or fabricate data. This is considered a serious form of misconduct and is designed to mislead others and damage the integrity of the scholarly record with wide-reaching and long-term consequences.
When submitting a manuscript to the journal, authors must ensure all data contained within their manuscript is accurate and correctly represents their work. To help assist the journal with manuscript evaluation, authors are expected to retain all raw data represented in their manuscripts.
If the original data cannot be produced on request, acceptance of a manuscript or published paper may be declined or retracted.
Research Data Policy and Data Availability Guidelines for Authors
Research Data Policy
The primary objective of the Management Dynamics Journal is to foster transparency, reproducibility, and the progress of knowledge within the academic community by promoting the sharing of research data. We acknowledge the significance of ensuring data availability to fellow researchers while upholding ethical and legal constraints. To achieve this objective, we have formulated the subsequent criteria for incorporating research data declarations in publications that are submitted.
Data Sharing Expectations:
Authors are advised to release their data to the public whenever feasible unless there are concerns regarding privacy, confidentiality, or legal restrictions. Data should be given in a manner that enables the verification of findings and the utilization of data for additional research.
Data Availability Statements
Authors must include a Data Availability Statement in their publications. This statement should provide explicit details on the location where the data supporting the study's findings may be obtained, or provide a clear explanation for why the data cannot be provided. Here are some instances of Data Availability Statements that are considered acceptable:
Data Available in a Public Repository:
Authors can deposit their datasets generated during and/or analyzed during the current study in a repository of their choice، to make their research data freely accessible, and enhance their discoverbility.
Data Available on Request:
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
No Data Available:
No datasets were generated or analyzed during the current study.
Desk rejection policy
- The topic/scope of the study is not relevant to the field of the Journal.
- There are publication ethics problems, non-adherence to international standard guidelines, and plagiarism (set at a similarity index of higher than 20 percent).
- The topic does not have a sufficient impact, nor does it sufficiently contribute new knowledge to the field.
- There are flaws in the study design.
- The objective of the study is not clearly stated.
- The study of the organization is problematic and/or certain components are missing.
- There are problems in writing or series infelicities of in the style of grammar.
- The manuscript does not follow the submission guidelines of the Journal.
Duplicate submission/publication
Authors are required to declare upon submission that the manuscript is not under consideration elsewhere, and as such the detection of a duplicate submission or publication is typically considered to be a deliberate act. This includes articles previously published in another language. For acceptable forms of secondary submissions or publications (e.g. an article translated into English), in accordance with ICMJE guidance, authors must seek permission from the publisher and copyright holder of the original article and must inform the Editor of the receiving journal about the history of the original article. It must also be made clear to readers that the article is a translated version, with a citation provided to the original article.
Funding
The journal requires that authors declare all the sources of funding including financial support in their manuscript. The authors should describe the role of the sponsor(s), if any, in any of the stages from study design to submission of the manuscript for publication. They should also state if the sponsor(s) had no such involvement. Please ensure that this information is accurate and in accordance with your funder’s requirements.
Images and figures
Photographs, video, or audio recordings that can reveal the identity of patients or study participants can only be included if they (or their next of kin if participants are deceased; parents or guardians if they are underage or considered to be vulnerable) have provided Consent to Publish.
Authors should be aware of any cultural sensitivities or restrictions associated with any images included in their manuscripts. For example, images of human remains or deceased humans is restricted in some cultures, and appropriate ethical guidelines should be adhered to by considering the views and approval processes of the affiliated communities.
Experimental photographic images including microscopy should accurately reflect the original image. Where images have been modified or enhanced in any way this must be stated with a full explanation within the manuscript as well as in the figure legend so as not to mislead readers about what the images show. Authors should be prepared to share the original, uncropped, unannotated, and unprocessed images with the journal editorial office upon request.
Please note that any modifications are only acceptable if these are minor in nature and have been applied to the whole image. Authors are required to include details of image-gathering methods and details of processes for any modifications made to images, including the name of the software (with version number) used. Any modifications which can alter the scientific interpretation of the image are not allowed.
Any images or figures that have been obtained from another published source can only be re-used if the authors have obtained the appropriate permissions for re-use from the copyright owner. A statement to confirm this must be included within the figure legend. The original source of the image must be cited, even in cases where the image or figure is not under copyright, or if re-use is allowed under a license that permits unrestricted re-use.
Misconduct
The journal takes all forms of misconduct seriously and will take all necessary action, in accordance with COPE guidelines, to protect the integrity of the scholarly record.
Examples of misconduct include (but are not limited to):
- Affiliation misrepresentation
- Breaches in copyright/use of third-party material without appropriate permissions
- Citation manipulation
- Duplicate submission/publication
- “Ethics dumping”
- Image or data manipulation/fabrication
- Peer review manipulation
- Plagiarism
- Text-recycling/self-plagiarism
- Undisclosed competing interests
- Unethical research
Duplicate Submission
Manuscripts that are found to have been published elsewhere, or to be under review elsewhere, will incur duplicate submission/publication sanctions. If authors have used their own previously published work, or work that is currently under review, as the basis for a submitted manuscript, they are required to cite the previous work and indicate how their submitted manuscript offers novel contributions beyond those of the previous work.
Citation Manipulation
Submitted manuscripts that are found to include citations whose primary purpose is to increase the number of citations to a given author’s work, or to articles published in a particular journal, will incur citation manipulation sanctions.
Data Fabrication and Falsification
Submitted manuscripts that are found to have either fabricated or falsified experimental results, including the manipulation of images, will incur data fabrication and falsification sanctions.
Improper Author Contribution or Attribution
All listed authors must have made a significant scientific contribution to the research in the manuscript and approved all its claims. It is important to list everyone who made a significant scientific contribution, including students and laboratory technicians.
Redundant Publications
Redundant publications involve the inappropriate division of study outcomes into several articles.
Image manipulation
Misconduct constitutes a violation of this editorial policy, journal policies, publication ethics, or any applicable guidelines/policies specified by COPE, WAME, ICMJE, and STM. Any other activities that threaten/compromise the integrity of the research/publication process are potential misconduct. Suspected cases of misconduct will be investigated according to COPE guidelines
Open Access Policy
Every peer-reviewed research article appearing in this journal will be published open access. This means that the article is universally and freely accessible via the internet in perpetuity, in an easily readable format immediately after publication. A CC user license manages the reuse of the article. All articles will be published under the following license:
CC-BY License
Articles published are made freely available online upon publication without subscription barriers to access. Users of such published articles are entitled to use, reproduce, disseminate, or display these articles provided that:
- The original authorship is properly and fully attributed;
- The journal and publisher are attributed as the original place of publication with correct citation details given;
- If an original work is subsequently reproduced or disseminated not in its entirety but only in part or as a derivative work this is clearly indicated.
Authors are also entitled to deposit the final electronic version of the article into an institutional or centrally organized subject repository upon publication. This is provided that they include a link to the published version of the article on the journal's website and that the journal and the journal are attributed as the original place of publication, with correct citations given.
Publication Ethics
Mansoura Veterinary Medical Journal follows ethical standards as prescribed by the Committee on Publication Ethics (COPE), and aims to aim to adhere to its guidelines and core practices
Authors
AUTHORS AFFIRM THE FOLLOWING
- Submitted manuscripts are original works of the author (s).
- The manuscript is not published or considered for publication elsewhere. Papers published at conferences are considered provided that this is declared in the cover letter.
- Submission of a manuscript to more than one journal at the same time is unethical.
- A conflict of interest statement should be provided.
- providing an acknowledgment to anyone who contributed to the research but is not an author.
- Communication with the editor is essential if any error is present in the paper after publication.
- Authors should use only citations that are relevant to their manuscripts. Authors providing citations whose primary purpose is to increase the number of citations to a given author’s work, or to articles published in a particular journal, may incur sanctions.
- Authors should avoid data Fabrication, manipulation, or falsification in their work. The Mansoura Vet Med J will follow the COPE guidelines in suspected cases.
- Authors should avoid plagiarism. The policy of Mansoura Vet Med J is to publish only unplagiarized material. Submitted manuscripts are checked for similarity index by TURNITIN. Highly similar articles are directly rejected. The plagiarism is counted excluding references.
Reviewers
Reviewers are expected to evaluate a manuscript for novelty and integrity. All manuscripts are reviewed in fairness based on the intellectual content of the article regardless of gender, race, ethnicity, religion, citizenship, or political values of the author(s).
- Any observed conflict of interest during the review process must be communicated to the Editor.
- All information concerning the manuscript is kept confidential.
- Any information that may be the reason for the rejection of publication of a manuscript must be communicated to the Editor.
Editors
Editors must confirm the following
- Regardless of gender, ethnicity, race, religion, citizenship, or political values of authors, all manuscripts are evaluated in fairness based on the scientific contents.
- Information pertaining to manuscripts is kept confidential.
- Any observed conflict of interest pertaining to manuscripts should be disclosed.
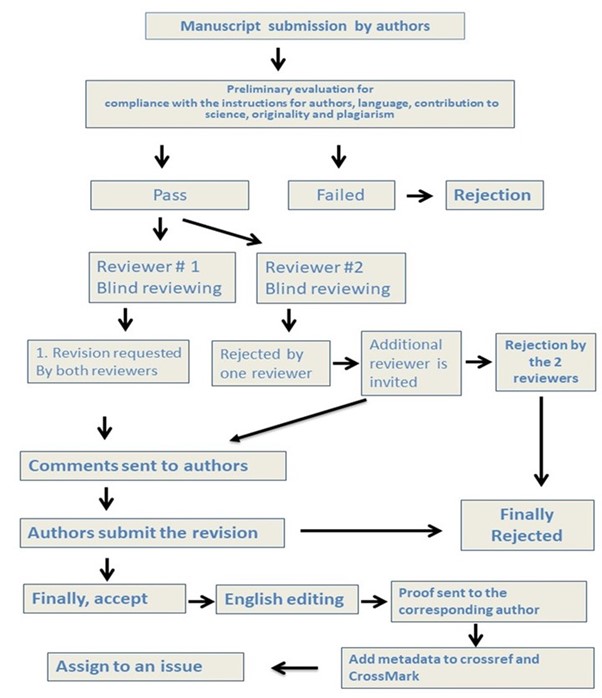
Peer review process
Type of Review: Submitted manuscripts are subjected to Double-blind Peer review.
Firstly, submitted manuscripts are subjected to preliminary evaluation for their novelty, compliance with the instructions for authors, and language. Manuscripts that are found as insufficient or non-compliant with the instructions for authors may be rejected without peer review. In addition, manuscripts are routinely subjected to plagiarism checks with standard software. The manuscript is directly rejected if plagiarism is detected.
Editors and referees who are expert researchers in their fields assess scientific articles submitted to Mansoura Veterinary Medical Journal (Mansoura Vet. Med. J.). A blind peer review strategy is applied to the evaluation process. The chief editor, if found necessary, may assign a Subject Editor for the article or may perform the scientific assessment of the article. Editors may also assign referees for the scientific assessment of the article and make their decisions based on reports by the referees. The Editor-in-Chief makes the final decision regarding the publishing of the article.
Authors have to review the instructions for submission and the steps for reviewing on the journal website https://mvmj.journals.ekb.eg/
The role of the editor:
The chief editor is responsible for monitoring all steps of the review process which should be fair, precise, and rapid. Mainly, the editor must do the following activities:
- Selecting manuscripts suitable for publication only.
- Ensuring a supply of high-quality manuscripts to the journal by identifying important “hot topics”.
- Increasing the journal’s impact factor and maintaining the publishing schedule.
- Providing strategic input for the journal’s development.
- Organizing the flow of manuscripts by communicating with the authors, referees, and publishers.
- Keeping the confidentiality of every author’s work.
- Describing, implementing, and regularly reviewing policies
- Dealing with all authors with fairness, courtesy, honesty, and transparency.
- Setting up a reliable panel of expert reviewers.
- Offering feedback to reviewers when required and ensuring that any feedback to authors is constructive.
Cancellations of the Submission
Papers may be returned to authors to prevent, double publication, prevent ethical breaches, and even if articles have been accepted or published, they can be withdrawn from publication if necessary. The Editors-in-Chief has the right to return or withdraw articles in the following conditions:
- The article is not within the scope of the journal
- The paper is published or considered for publication in other journals
- The scientific quality and content of the article do not meet the standards of the journal.
- The article was not prepared in compliance with scientific publication ethics
- High similarity to another work is detected by the plagiarism detection software.
- The authors do not perform the requested corrections within the requested time.
- An author is added/removed, the order of the authors is changed, the corresponding author is changed, or the addresses of the authors are changed without the consent of the Editor-in-Chief.
- A statement is not submitted indicating the approval of the ethics committee.
- Human rights or animal rights are violated.
- Data used in the study cannot be provided upon request.
DECISION
The decision may be any one of the following:
- Accept (with or without editorial revision).
- Reject with the possibility of resubmission: The authors are informed that further work might justify a resubmit.
- Reject, typically on grounds of specialist interest, lack of novelty, insufficient conceptual advance, or major technical and/or interpretational problem.
Peer review of an article is expected to be completed within 4 weeks. The decision by the Editor-in-Chief is given in approximately 6 weeks. If the corresponding author does not submit the revised version within 3 weeks of sending the revision letter, the article will be considered rejected, and the article metadata will be deleted from the system. If the corresponding author does not respond within 2 weeks after accepting the manuscript, the article will be considered rejected, and the article metadata will be deleted from the system.
SCHEMATIC DIAGRAM FOR STEPS OF REVIEW
Plagiarism
The journal has a strict policy against plagiarism. All submitted manuscripts are checked for plagiarism using professional plagiarism-checking software. Submitted manuscripts with an unacceptable similarity index resulting from plagiarism are rejected immediately.
This applies to data, images, words or ideas taken from any materials in electronic or print formats without sufficient attribution. This can include abstracts, seminar presentations, laboratory reports, thesis or dissertation, research proposals, computer programs, online posts, grey literature, and unpublished or published manuscripts.
The use of any such material either directly or indirectly should be properly acknowledged in all instances and the source of content must always be cited.
The journal uses plagiarism-checking to screen all submitted manuscripts and will deal with cases of plagiarism according to COPE guidelines. Any manuscript found to contain plagiarized material will not be considered for publication.
Preprints policy
Authors can share their preprint anywhere at any time. If accepted for publication, we encourage authors to link from the preprint to their formal publication via its Digital Object Identifier (DOI). Authors can update their preprints on arXiv or RePEc, etc. with their accepted manuscript.
Protection of Patients' Rights to Privacy
Identifying information should not be published in written descriptions, photographs, sonograms, CT scans, etc., and pedigrees unless the information is essential for scientific purposes and the patient (or parent or guardian, wherever applicable) gives informed consent for publication. Authors should remove patients' names from figures unless they have obtained informed consent from the patients.
The journal abides by ICMJE guidelines:
- Authors, not the journals nor the publisher, need to obtain the patient consent form before the publication and have the form properly archived. The consent forms are not to be uploaded with the cover letter or sent through email to editorial or publisher offices.
- If the manuscript contains patient images that preclude anonymity or a description that has an obvious indication of the identity of the patient, a statement about obtaining informed patient consent should be indicated in the manuscript.
Research ethics and consent
Studies in Humans, Animals, and Plants
All original research papers involving humans, animals, plants, biological material, protected or non-public datasets, collections, or sites, must include a written statement under an Ethics Approval section including the following:
- The name of the ethics committee(s) or institutional review board(s) involved.
- The number or ID of the ethics approval(s).
- A statement that human participants have provided informed consent before taking part in the research.
Research involving animals must adhere to ethical standards concerning animal welfare. All original research papers involving animals must:
- Follow international, national, and institutional guidelines for the humane treatment of animals.
- Receive approval by the ethics review committee at the institution or practice at which the research was conducted and provide details on the approval process, names of the ethics committee(s) or institutional review board(s) involved, and the number or ID of the ethics approval(s) in the Ethics Approval section.
- Provide justification for the use of animals and the species selected.
- Provide information about housing, feeding, and environmental enrichment, and steps taken to minimize suffering.
- Provide mode of anesthesia and euthanasia.
Research that does not meet the above-listed requirements regarding ethical approval and animal welfare will be rejected.
Studies in humans and animals
If the work involves the use of human subjects, the author should ensure that the work described has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. The manuscript should be in line with the Recommendations for the Conduct, Reporting, Editing and Publication of Scholarly Work in Medical Journals and aim for the inclusion of representative human populations (sex, age, and ethnicity) as per those recommendations. The terms sex and gender should be used correctly.
Authors should include a statement in the manuscript that informed consent was obtained for experimentation with human subjects. The privacy rights of human subjects must always be observed.
All animal experiments should comply with the ARRIVE guidelines and should be carried out in accordance with the U.K. Animals (Scientific Procedures) Act, 1986 and associated guidelines,EU Directive 2010/63/EU for animal experiments, or the National Research Council's Guide for the Care and Use of Laboratory Animals and the authors should clearly indicate in the manuscript that such guidelines have been followed. The sex of animals must be indicated, and where appropriate, the influence (or association) of sex on the results of the study.
Informed consent
Patients have a right to privacy that should not be violated without informed consent. Identifying information, including names, initials, or hospital numbers, should not be published in written descriptions, photographs, or pedigrees unless the information is essential for scientific purposes and the patient (or parent or guardian) gives written informed consent for publication. Informed consent for this purpose requires that an identifiable patient be shown the manuscript to be published. Authors should disclose to these patients whether any potentially identifiable material might be available via the Internet as well as in print after publication. Patient consent should be written and archived either with the journal, the authors, or both, as dictated by local regulations or laws. Nonessential identifying details should be omitted. Informed consent should be obtained if there is any doubt that anonymity can be maintained. For example, masking the eye region in photographs of patients is inadequate protection of anonymity. If identifying characteristics are altered to protect anonymity, such as in genetic pedigrees, authors should provide assurance, and editors should so note, that such alterations do not distort scientific meaning. When informed consent has been obtained, it should be indicated in the published article.
Research involving plants
Studies on plants must be carried out within the guidelines provided by the authors’ institution and national or international regulations. Where applicable, a statement of permissions granted or licenses should be included. Authors should comply with the Convention on Biological Diversity and the Convention on the Trade in Endangered Species of Wild Fauna and Flora.
Standards of reporting
Research should be communicated in a way that supports verification and reproducibility, and as such, we encourage authors to provide comprehensive descriptions of their research rationale, protocol, methodology, and analysis.
Use of third-party material
Authors must obtain the necessary permission to reuse third-party material in the article. The use of short extracts of text and some other types of material is usually permitted, on a limited basis, for the purposes of criticism and review without securing formal permission. If authors wish to include any material in their paper for which they do not hold copyright, and which is not covered by this informal agreement, they will need to obtain written permission from the copyright owner prior to submission. For more information on requesting permission to reproduce work(s) under copyright please send an email to [email protected]






